Colin Banas, M.D., M.H.A.
What’s Needed for EPCS: Critical Requirements for Providers and Vendors
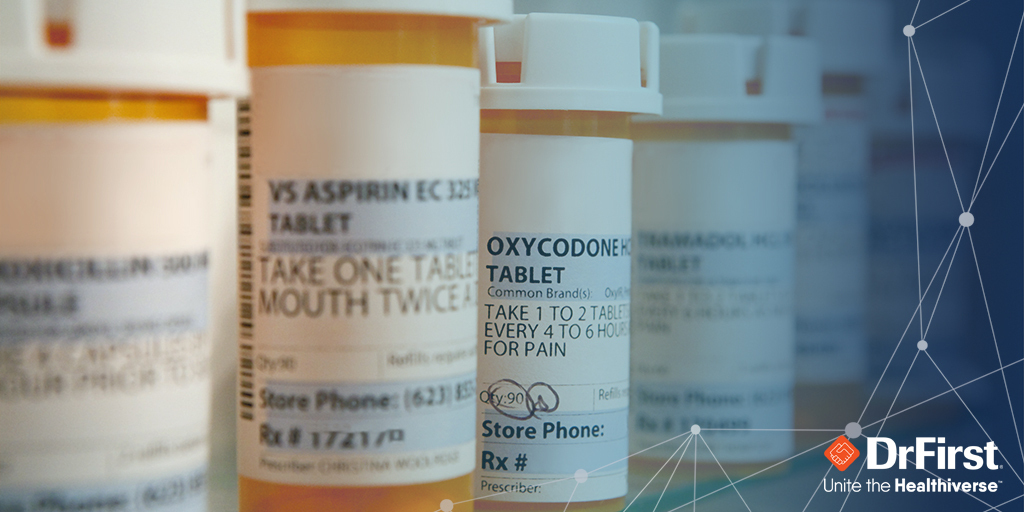
E-prescribing of controlled substances (EPCS) has grown rapidly over the past ten years, not only because technology has improved but also due to the substantial benefits for patients and physicians. Every state in the U.S. now allows EPCS, with many states mandating its use along with prescription drug monitoring programs (PDMPs).
E-Prescribing of Controlled Substances: A Look Back
EPCS was first developed in 2008, when DrFirst worked with the Massachusetts Department of Health to introduce the first controlled substance e-prescribing solution in the country. Supported by a multi-year grant from the U.S. Department of Health & Human Services and the Agency for Healthcare Research and Quality, and conducted under a waiver from the DEA, the pilot program became EPCS Gold® by DrFirst. In 2010, the Drug Enforcement Administration (DEA) released its interim final rule permitting use nationally, subject to state regulation.
With the opioid epidemic officially declared a public health emergency in 2017, EPCS and state-based PDMPs became vital to helping prevent doctor shopping and prescription fraud, as well as abuse and diversion that has contributed to the opioid epidemic.
EPCS Requirements
When the DEA approved EPCS, it established strict requirements which, except for minor tweaks over the years, remain unchanged today.
First, the EPCS application’s user interface must display the following information to the prescriber at signing: patient, prescriber, prescription, pharmacy information, and signing statement. Second, prescribers must undergo identity proofing (IDP) based on NIST requirements to identify the prescriber using two of three authentication factors:
- Who you are (a biometric, such as a fingerprint or iris scan)
- What you know (a password), and/or
- What you have (a token)
Third, the e-prescribing system must be able to grant “access control,” a process that involves two people and tells the e-prescribing system or EHR that this prescriber is approved to use it for EPCS. Finally, the e-prescribing system or EHR must undergo an extensive audit to ensure that each of the DEA requirements are addressed.
Modernizing EPCS Rules
Ten years after the DEA started permitting e-prescribing of controlled substances, EPCS still lags e-prescribing in general, with only about 38% of prescriptions for controlled substances processed electronically, compared with 80% of non-controlled substances.
In a conversation with John Lynn of Healthcare IT Today, DrFirst President Cam Deemer explained that the DEA requirements for EPCS have proved so onerous for some physicians, many simply stopped prescribing certain controlled substances. Others are writing these prescriptions by hand. (Watch the Healthcare IT Today video interview on modernizing EPCS regulations.)
While the strict rules for EPCS are understandable in light of the legal issues of diversion and fraud that contribute to the opioid crisis, it’s important to recognize that a lot has changed in terms of technology and security in the past decade. Fortunately, the DEA has reopened the comment period to solicit input before publishing its final rule on EPCS.
In response, DrFirst is urging the DEA to update its requirements in a way that addresses the need for security and fraud-prevention, while also recognizing healthcare providers’ needs for a seamless e-prescribing workflow. Updating the requirements is vital to easing the process for physicians and promoting wider adoption of EPCS.
The January 1, 2021, federal mandate requiring e-prescribing for all controlled substances under Medicare’s Part D drug plan also will encourage use of EPCS.
In this evolving environment, it’s more important than ever to keep up to date on the latest e-prescribing requirements and solutions. As the pioneer of the technology for EPCS and creator of the leading mobile e-prescribing app iPrescribe®, DrFirst is uniquely positioned to help your practice prepare for success. Take a look at our medication management solutions and let us know how we can help.